Available for download as a PDF:
1. Introduction to NICHD Policy for the Data and Specimen Hub
Given its mission to improve public health through research, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) believes that the full value of research can be realized if research data and biospecimens from NICHD-funded research studies are made available as rapidly as possible to the research community. NICHD expects intramural and extramural-supported investigators to share research data and research resources for broader dissemination in compliance with related NIH Sharing Policies. To facilitate sharing and access of data and specimens from human subjects’ research, NICHD has established the NICHD Data and Specimen Hub (DASH).
NICHD strongly encourages research investigators funded by the Institute to use NICHD DASH to share research data. In addition, certain studies will also be collecting biospecimens to be stored in the NICHD Contracted Biorepository, henceforth referred to as NICHD Biorepository, and ultimately shared with other investigators.[Note: the NICHD Biorepository does not store biospecimens from all NICHD-supported studies; some are stored in other external biorepositories.] NICHD expects that new research data generated from biospecimens obtained from the NICHD Biorepository and citations of resulting publications from the use of research data and/or biospecimens will be deposited back into DASH and made available to other investigators.
NICHD recognizes that databases and repositories may be established by other entities to achieve different scientific aims or to integrate different analytic capabilities for the purpose of sharing. The NICHD DASH Policy is not intended to constrain the development of such databases and repositories nor curtail depositing research data or biospecimens to them (as may be appropriate or required for some research programs). However, NICHD encourages investigators to submit data to DASH, as DASH serves as a resource to ensure that research data and any biospecimens collected from studies supported by NICHD are available to the broader research community for secondary use.
A goal for DASH is to provide a catalog of the storage locations of data and biospecimens from all NICHD-funded studies, even if not deposited in DASH and/or the NICHD Biorepository. This would allow NICHD DASH to provide a URL link to research data and biospecimen storage locations for all NICHD-funded research studies
2. Principles of the Policy
Sharing data and biospecimens ensures that NICHD is a responsible steward of the public funds entrusted to them. Rapid and broad research data and biospecimen access is particularly important because of the extraordinary opportunities for making comparisons across multiple studies as well as generating new scientific hypotheses and results. Another core principle of this policy is to ensure adequate protections for participants and their privacy.
3. Applicability of the Policy
NICHD supports the sharing in DASH of research data and biospecimens derived from NICHD-funded research studies that are:
- Permissible given the consent provided by research subjects
- In alignment with NIH Data Sharing Policies
- In alignment with the criteria listed below for sharing research data and specimens
NICHD DASH Policy and Procedures apply to three scenarios:
- NICHD-funded investigators who want to share research data in accordance with NIH Data Sharing Policies.
- Qualified investigators who want to access research data stored in NICHD DASH.
- Qualified investigators who want to request biospecimens stored in the NICHD Biorepository. NICHD DASH serves as the portal for accessing specimens stored in the NICHD Biorepository. It should also be noted that the NICHD Biorepository is not a comprehensive repository of biospecimens from all NICHD-funded or conducted studies.
4. NICHD DASH Policy Considerations
Several factors are considered by NICHD in the implementation of the DASH Policy and Procedures.
4.1 Protection of Research Participants
Protection of research participants is essential for conducting biomedical research. NICHD is committed to responsible stewardship of research data and biospecimens throughout the research process, including data and biospecimen sharing; such stewardship is necessary for protecting the interests of study participants and to maintaining public trust in biomedical research.
4.1.1 De-identification of Research Data and Biospecimen Catalog
4.1.1.1 Research Data De-identification
To ensure that the identities of research subjects cannot be readily ascertained with the research data, NICHD DASH will store only research data that are without identifiers and coded by the submitting institution/investigator. NICHD will not hold direct participant identifiers for research data stored within NICHD DASH, nor will NICHD have access to any identifying links between the data and the participants that may reside with the primary investigators and institutions for particular studies. (Refer to Section 6.1.1 for additional information on research data and biospecimen catalog de-identification guidance for investigators submitting to DASH.)
4.1.1.2 Biospecimen Catalog De-identification
NICHD DASH is not involved, directly or indirectly, in the storing of biospecimens in the NICHD Biorepository but serves as the portal for accessing biospecimens deposited in the NICHD Biorepository. NICHD DASH will provide a de-identified biospecimen catalog for each study, which lists the biospecimens available from that study in the NICHD Biorepository (or other biorepository, if provided) and has its research data archived in NICHD DASH.
Exception: For a limited number of older studies (including complete and active studies) in the NICHD Biorepository, the biospecimen catalog is not coded using the same unique de-identified code as the research data stored in NICHD DASH. Refer to Section 6.1.1 for details on what an investigator needs to do to address this issue. NICHD DASH will not provide to any investigators access to the biospecimen catalog containing individually identifiable information or the cross-walk file linking the code to the individually identifiable information stored in DASH.
4.1.2 IRB Review of Research Data and Biospecimen Catalog Submission or Access
The NICHD DASH Policy and Procedures include steps to protect the interests and privacy concerns of individuals, families, and groups who participate in research. In Section 6.1.2, there is a description of what Institutional Review Board (IRB) approvals and other related information should be provided to NICHD DASH.
4.1.3 Non-research Use of Data
As an agency of the Federal Government, there may be instances where NICHD is required by law to release government records. For example, NICHD is required to release government records in response to a request under the Freedom of Information Act (FOIA), unless those records are specifically exempted from release.
4.1.4 Stigmatization of Research Data
Some research data to be included in NICHD DASH or the research data resulting from the analysis of specimens may suggest the existence of either individual traits or socially stigmatized traits. Analytical tools are increasingly able to make inferences about individual traits (e.g., height; weight; and skin, hair, and eye color) and to suggest predilections for characteristics (e.g., risk of developing some diseases) and behaviors with social stigma.
In recognition of these concerns, NICHD DASH requires all study investigator institutions submitting research data to DASH to obtain approval from an IRB and/or equivalent Privacy Board (refer to Section 6.1.2). In the event that research data or biospecimen access requests raise questions or concerns related to privacy, confidentiality, risks to populations or groups, or other relevant topics, the DASH Committee will consult with other NIH staff experts as appropriate before making a final decision on release of research data and/or biospecimens to requesters.
4.1.5 Informed Consent
Adequate consent for research data and biospecimen sharing requires participants to understand both the risks and potential benefits of the proposed sharing. Key stakeholders in these considerations are: research participants who have participated in studies; investigators developing informed consent processes; institutions approving the submission of research data to NICHD DASH or access to biospecimens from the NICHD Biorepository; and IRBs asked to review studies proposing broad research data and specimen sharing.
NICHD recognizes that the ethical considerations relevant to research data and biospecimen sharing are complex and dynamic. Participant interests extend beyond individual participants to families, communities, and broader cultural contexts. Furthermore, especially complex issues exist where participant consent has been provided by proxy (e.g., pediatric research or studies involving mental health disorders). These broad interests should be considered when submitting research data and biospecimens to NICHD DASH and/or the NICHD Biorepository. NICHD will also consider these issues on an ongoing basis when sharing data and/or biospecimens.
In general, research data and biospecimen catalogs submitted to NICHD DASH will be from studies where data collection is complete and the study is ready to be shared as per NIH Sharing Policies. For certain studies, NICHD anticipates variation in the extent to which research data and biospecimens can be shared based on how it is specified in the informed consent. This context will be considered when NICHD is reviewing access requests and may limit how data or biospecimens can be shared.
4.1.6 Return of Results to Participants
NICHD will not provide study participants with results of secondary analyses from research data or biospecimens accessed from NICHD DASH. Secondary research data or biospecimen users will not have access to identifying information; consequently, NICHD DASH users will not be able to return individual results directly to the subjects.
4.2 Funding Status
Given that biospecimens are a finite resource, investigators interested in requesting access to biospecimens from the NICHD Biorepository must ensure that they have funding available to conduct the proposed study or studies. This should also include funds to obtain the specimens from the NICHD Biorepository.
4.3 Scientific Publication
The nature of NICHD DASH dictates that the research data and biospecimens deposited from research studies are available for meaningful secondary analysis to investigators who request the research data and/or biospecimens, and that all results from such use are under the purview of the requesting investigators without restrictions for publication. NICHD expects all investigators who access research data and biospecimens from NICHD DASH to acknowledge the contributing investigator(s) who conducted the original study, the funding organization(s) that supported the original study, and NICHD DASH in all resulting oral or written presentations, disclosures, or publications of the analyses. Guidance for acknowledgement text will be provided in the NICHD DASH Data Use Agreement and/or the NICHD DASH Material Transfer Agreement to be signed by the requesting investigators and their authorized institutional business official.
4.4 Intellectual Property
NICHD encourages development of new diagnostics, therapeutics, or other interventions building on basic discoveries enabled through research data obtained from NICHD DASH and biospecimens from the NICHD Biorepository. Data and biospecimen recipients are free to pursue patent protection on any inventions or discoveries developed through their analysis and use of NICHD DASH research data and biospecimens.
NICHD expects that findings identified through NICHD-funded research studies and their obvious implications will remain available to all investigators, unencumbered by intellectual property claims. NICHD discourages premature claims on potential pre-competitive information that may impede research, although it encourages patenting of technology suitable for subsequent private investment that may lead to the development of products that address public needs.
5. Governance and Oversight of NICHD DASH
NICHD recognizes that scientific, ethical, and societal issues relevant to the NICHD DASH Policy and Procedures Statement are evolving. NICHD established a governance structure that:
- Ensures ongoing institutional oversight for NICHD DASH, including adequate quality control and security measures for research data archiving and research data and/or biospecimen use practices;
- Obtains regular input from public representatives, including those with expertise in bioethics, privacy, data security, and appropriate scientific and clinical disciplines; and
- Will revisit and revise the NICHD DASH Policy and Procedures Statement as appropriate and in accordance with any new or updated NIH or other federal policies and guidance that impact research data and biospecimen sharing.
The NICHD Director and/or designee oversees the NICHD DASH Policies and Procedures. The NICHD Director established a NICHD DASH Committee composed of NICHD staff with the appropriate expertise to provide input to the Director. The Committee provides oversight of the management and performance of NICHD DASH. Specialized sub-committees related to research data submission, research data and biospecimen access (such as the Data/Biospecimen Access Committee), and quality control report to the DASH Committee. To maintain policies consistent with evolving technological and ethical considerations, the NICHD Director solicits, on an as-needed basis, recommendations on the NICHD DASH Policy Statement from external experts representing public and scientific stakeholders through the National Advisory Child Health and Human Development (NACHHD) Council.
The NICHD DASH Committee established and maintains operating policies and procedures for NICHD DASH to address issues including, but not limited to:
- Privacy and confidentiality of research participants
- Interests of individuals and groups
- Data and biospecimen catalog submission procedures
- Data and biospecimen access and use procedures
- Data security mechanisms based on the content and level of risk
6. Data and Biospecimen Catalog Submission and Access Requirements
This section expands upon the NICHD DASH Policy Statement with additional details on the specific procedures for submitting and accessing research data and biospecimen catalogs in DASH.
6.1 Data and Biospecimen Catalog Submission Requirements
Extramural and intramural investigators submitting study research data and the biospecimen catalog (which contains information about biospecimens, such as type, amount available, participant age, etc., from a particular study that are available for sharing; see Section 8, Glossary) to NICHD DASH must comply with the specific procedures described below.
NICHD-funded investigators who do not submit their research data to NICHD DASH are strongly encouraged to provide the name of the alternate publicly accessible data archive where the data are being stored to the appropriate NICHD Program Officer responsible for the study and are encouraged to justify why submission to the alternate archive is more suitable. Similarly, NICHD-funded investigators submitting biospecimens to a biorepository other than the NICHD Biorepository for those studies with research data archived in NICHD DASH are also strongly encouraged to provide the name of the biorepository to NICHD DASH.
6.1.1 De-identified Data and Biospecimen Catalog
The data and biospecimen catalog submitted to NICHD DASH must be stripped of identifiers and coded based on the following criteria (Refer to Section 4.1.1):
- The identities of research subjects cannot be readily ascertained or otherwise associated by NICHD DASH staff or secondary data users (45 C.F.R. 46.102(e)).
- The 18 identifiers1 enumerated at section 45 C.F.R. 164.514(b)(2) (the HIPAA Privacy Rule) are removed.
- The submitting institution has no knowledge that the remaining information could be used alone or in combination with other information to identify subjects.
- The biospecimen catalog (if applicable) must be coded with the same unique de-identified code used for the research data to enable linking of de-identified data to specimens from the same study participant.
- A cross-walk file linking the de-identified codes to the individually identifiable information will be retained by the submitting institution.
- Exception for biospecimen catalog (as determined by the DASH Committee and stated in Section 4.1.1.2): For certain studies, specifically older studies and as determined by the DASH Committee, where the data are de-identified and coded by the investigator but not the biospecimen catalog, investigators must submit to NICHD DASH the cross-walk file linking the de-identified codes to the individually identifiable information so that DASH can link the data with biospecimens.NICHD DASH will not provide to any investigator access to the biospecimen catalog containing individually identifiable information or the cross-walk file linking the code to the individually identifiable information stored in DASH.
- Investigators should refer to the NICHD DASH Data and Biospecimen Catalog De-identification Guidance when preparing research data and biospecimen catalogs for submission to NICHD DASH.
6.1.2 Institutional Certification
A submitting institution must certify whether a study-specific dataset is appropriate for submission to NICHD DASH and, when there are corresponding biospecimens stored in the NICHD Biorepository, whether they can be shared. This may necessitate consultation with an IRB and/or Privacy Board. All research data and biospecimen catalog submissions to NICHD DASH must be accompanied by an Institutional Certification from responsible Institutional Official(s) of the submitting institution stating that an IRB or equivalent Privacy Board has determined that sharing of data via DASH is consistent with the informed consent and that the identities of research participants will not be disclosed to NICHD. For exceptions where NICHD will retain the cross-walk file linking the code with individually identifiable information in the biospecimen catalog, submitting investigators will be required to provide an approval from the study’s IRB and/or an equivalent Privacy Board stating that they agree to submitting individually identifiable information to NICHD DASH.
At a minimum, the Institutional Certification must assure that:
- The research data and biospecimens were collected in a manner consistent with 45 C.F.R. Part 46.
- The research data and biospecimen catalog submission is consistent with all applicable Federal and state laws and regulations2, as well as relevant institutional and study policies.
- The investigator has de-identified the study research data and biospecimen catalog in compliance with the standards outlined in the NICHD DASH Policy (i.e., stripped of all 18 HIPAA identifiers).
- The identities of research participants will not be disclosed to NICHD DASH.
- The appropriate research uses of the research data and biospecimens and their limitations that are consistent with the informed consent documents are delineated during the process of research data and biospecimen catalog submission to NICHD DASH.
- The submitter has considered the risks to individuals, their families, and groups or populations associated with research data and biospecimen sharing via NICHD DASH.
- The investigator will be responsible to inform NICHD DASH if research data and biospecimen catalog need to be removed from the Archive for any reason, such as change in informed consent.
- An IRB and/or Privacy Board, as applicable, reviewed and verified that:
- 8.1 The submission to NICHD DASH and subsequent sharing for research purposes are consistent with the informed consent of study participants from whom the research data and biospecimens were obtained3.
- 8.2 For biospecimen catalog submission exceptions, if applicable, the cross-walk file linking the de-identified codes to the individually identifiable information can be shared with NICHD DASH.
NICHD envisions three potential mechanisms by which to obtain IRB and/or Privacy Board approvals – these mechanisms are not mutually exclusive:
- Completed studies that have active IRB review or are under continuing IRB review: Investigators to obtain approval from the study IRB.
- Completed studies that do not have an active IRB: Investigators to obtain approval or exemption from NICHD IRB or NIH Office of Human Subjects Research Protections (OHSRP) or submitting institution’s IRB or external commercial IRBs.
- NICHD DASH to operate under a formal NICHD IRB approved protocol to cover research data and biospecimen management activities for selected studies, including research data submissions and both research data and/or biospecimen requests.
In the event that a research participant withdraws consent to the submitting institution for future sharing of his/her individual-level research data that was submitted to NICHD DASH and, if applicable, any biospecimens stored in the NICHD Biorepository, the submitting institution will be responsible for informing NICHD DASH. Once informed, NICHD DASH will remove the entire set of research data or biospecimen catalog for the study with the participant in question. NICHD DASH will then request the submitter to furnish a redacted version of the research data and the biospecimen catalog as replacement for what was originally submitted to NICHD DASH.
NICHD will also terminate the NICHD DASH Data Use Agreement or amend the NICHD DASH Material Transfer Agreement established with research data and biospecimen recipients and instruct the recipients to destroy the research data or biospecimens in question so long as the recipients have not already begun using the research data and/or biospecimens. NICHD DASH will inform previous research data and biospecimen catalog recipient(s) if and when a redacted research data and biospecimen catalog is made available to NICHD DASH.
6.1.3 Data Quality and Documentation
Data submitted to NICHD DASH must be individual-level research data that is de-identified and usable for secondary analysis. All research data submitted to NICHD DASH must be accompanied by proper documentation to ensure meaningful use of the research data and to prevent misuse, misinterpretation, and confusion. Documentation provides information about the methodology and procedures used to collect the research data, details about research data variable codes, definitions of variables, variable field locations, frequencies, etc. The precise content of documentation will vary by scientific area, study design, type of research data collected, and characteristics of the research data. Examples of documentation files include protocols, codebooks, data dictionaries, data collection instruments, methods for data cleaning, data analysis plans, summary statistics, project summaries, and bibliographies of publications pertaining to the data. While investigators may submit all the aforementioned information along with the research data, the following four documents are required for submission of research data in NICHD DASH:
- Study Protocol
- Data Collection Instruments
- Data Dictionary/Codebook
- Data De-identification Methodology
6.1.4 Data or Biospecimen Catalog Withdrawal and Resubmission
In the event that research data or biospecimen catalog submitted to NICHD DASH must be modified or corrected, such action is the sole responsibility of the submitting investigator and their organization. Please refer to Section 6.1.2 for further guidance on research data withdrawal and resubmission.
6.2 Data and Biospecimen Access Requirements
NICHD DASH will serve as the portal for accessing research data stored in it and, if available, corresponding biospecimens stored in the NICHD Biorepository. Investigators interested in obtaining research data and biospecimens through NICHD DASH must comply with the specific requirements listed below.
6.2.1 Data Use Agreement and/or Material Transfer Agreement
Data and biospecimen access requests should include a brief description of the proposed use of the research data and biospecimens (if applicable). Requesting investigators will be required to sign a NICHD DASH Data Use Agreement for research data as well as the NICHD DASH Material Transfer Agreement for biospecimens, whereby they agree to:
- Use the research data or biospecimens only for the research plan or non-research use approved by the NICHD DASH Data and Biospecimen Access Committee
- Not share research data or biospecimens with individuals other than those listed in the request and subsequent agreement
- Protect research data and biospecimen confidentiality
- Not attempt to identify individual participants from whom research data or biospecimens were obtained
- Follow appropriate research data and biospecimen security protections (e.g., physical security, information technology security, user training)
- Follow all applicable laws, regulations, and local institutional policies and procedures for handling research data and biospecimens
- Not sell any of the research data or biospecimens
- Agree to report violations of the NICHD DASH Data Use Agreement and/or the NICHD DASH Material Transfer Agreement to NICHD DASH
- Acknowledge the contribution of the research data and biospecimen submitters and NICHD DASH with regard to publication and intellectual property
- Provide annual progress reports to NICHD on use of the research data and biospecimens
6.2.2 IRB Approval
To access research data and/or biospecimens (if available), an approval, exemption, or declaration that the research does not involve human subjects may be required from the requester’s IRB, as specified by the research data or biospecimen submitter. The requirement for an IRB approval or exemption will be delineated in the research data and biospecimen request form provided by NICHD DASH. The IRB approval or exemption must be submitted at the time of the request for data and/or biospecimens to NICHD DASH.
6.2.3 Funding for Data and Biospecimens
There is no cost associated with accessing data in DASH.
Various costs incurred by the investigators for obtaining biospecimens include, but are not limited to, sample validation pulls, preparing aliquots, biospecimen relabeling, and shipping. NICHD DASH will not provide any financial assistance to access biospecimens from the NICHD Biorepository.
As noted above in Section 6.2, investigators requesting biospecimens must demonstrate availability of funding prior to initiating a material transfer. If investigators do not have funding but require proof of availability of the biospecimens to apply for research funding, they must submit a request to NICHD DASH for a Letter of Biospecimen Availability, which will be based on the catalog provided to NICHD DASH by biospecimen submitters. Biospecimens will not be held by the NICHD Biorepository for investigators based on the Letter of Biospecimen Availability, and requesting investigators must submit an updated request with proof of funding to NICHD DASH after securing research funding.
6.2.4 NICHD DASH Data and Biospecimen Access Committee Approval
The NICHD DASH Data and Biospecimen Access Committee will review requests for research data and/or biospecimens to determine whether the proposed use is scientifically and ethically appropriate and does not conflict with constraints or research data/biospecimen use limitations identified by the institutions that submitted the research data and biospecimens. Certain studies also require additional approvals from a study-specific entity, such as the study Steering Committee or Principal Investigator. In such cases, the NICHD DASH Data and Biospecimen Access Committee will contact and obtain approval from these entities prior to reviewing and approving the request.
7. Inquiries
For inquiries on the NICHD DASH Policy Statement, please send an email to supportdash@mail.nih.gov.
8. Glossary
Biospecimens refers to biological specimens and/or derivatives collected from participants in research studies that have data available through NICHD DASH. Biospecimens are subject to release to the recipient, according to the criteria laid out in this NICHD DASH Policy, which includes the execution of a NICHD DASH MTA, approval by the NICHD DASH Biospecimen Access Committee, and, if applicable, by study-specific entities.
Biospecimen Catalog refers to information about biospecimens (such as type, amount available, participant age, etc.) from a particular study that are available for sharing.
Coded: With respect to private information or human biological specimens, coded means that:
- Identifying information (such as name or social security number) that would enable the investigator to readily ascertain the identity of the individual to whom the private information or specimens pertain has been replaced with a number, letter, symbol, or combination thereof (i.e., the code); and
- A key to decipher the code exists, enabling linkage of the identifying information with the private information or specimens.
De-identified data are participant data that formerly contained individually identifiable information, but which has had all uniquely identifying information, numbers, characteristics, and codes removed such that the data cannot be used alone or in combination with other information to identify or readily ascertain the identity of the individual who is the subject of the data (45 CFR 164.514, 45 CFR 46.102(e), 45 CFR 46 Subpart A). Individually identifying information includes, but is not limited to, the 18 categories of identifiers described in 45 CFR 164.514(b)(2) and any other information that can be used to readily ascertain an individual as described in 45 CFR 46.102(e).
Research Data refers to data and information collected and recorded from participants in research studies that are available through NICHD DASH. Research data includes recorded factual material commonly accepted in the scientific community as necessary to validate research findings. It does not include preliminary analyses; drafts of scientific papers; plans for future research; peer reviews; communications with colleagues; physical objects (e.g., laboratory samples, audio, or video tapes); trade secrets; commercial information; materials necessary to be held confidential by a researcher until publication in a peer-reviewed journal; information that is protected under the law (e.g., intellectual property); personnel and medical files and similar files, the disclosure of which would constitute an unwarranted invasion of personal privacy; or information that could be used to identify a particular person in a research study.
Requester is an investigator who is requesting research data or biospecimens from NICHD DASH.
Submitter is an investigator who has deposited research data to NICHD DASH or biospecimens to the NICHD Biorepository for sharing via DASH.
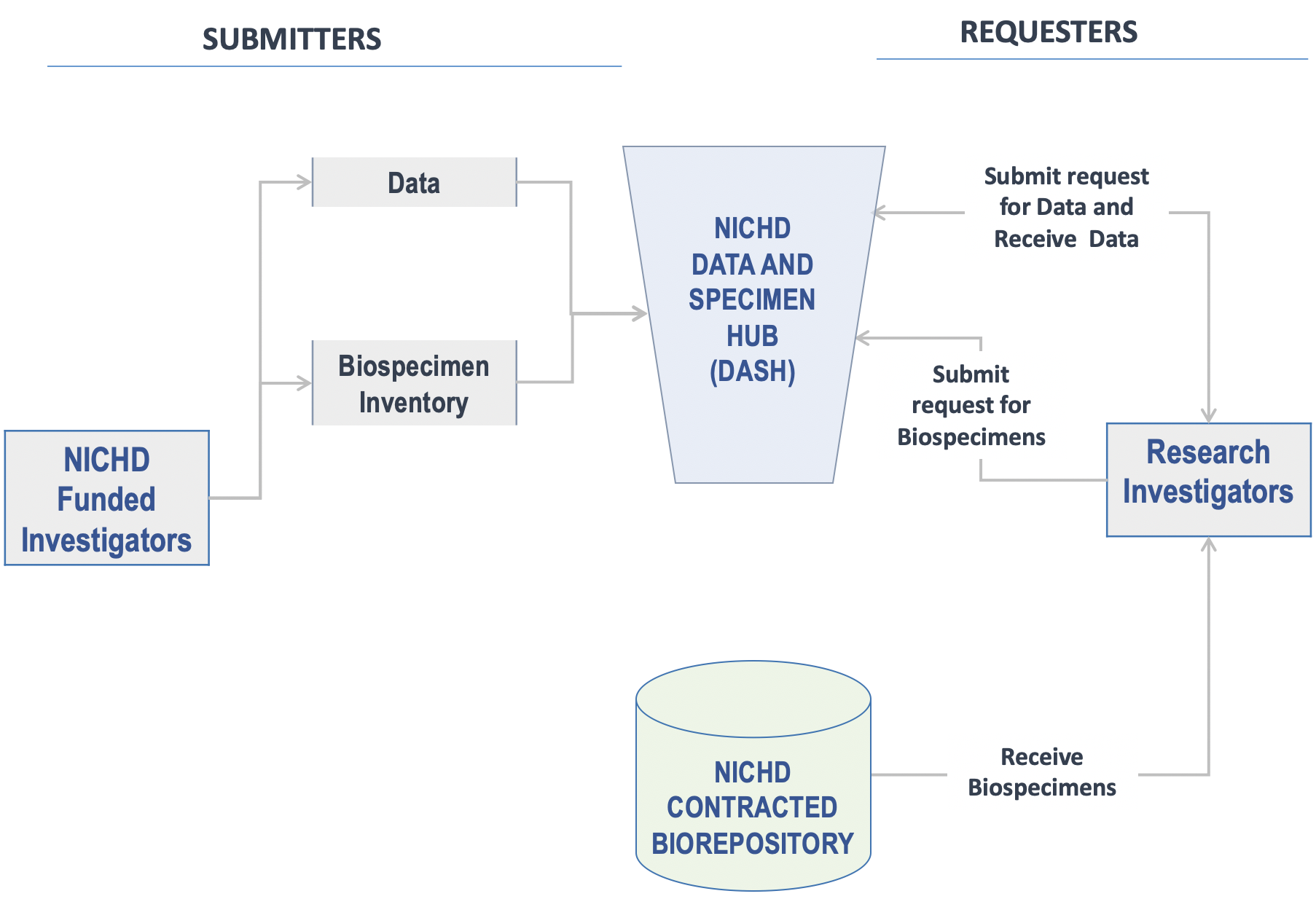
9. Appendix: NICHD DASH - Data and Biospecimen Submission and Request Overview
1 The identities of research subjects cannot be readily ascertained or otherwise associated with the data by the repository staff or secondary data users (Common Rule); and the following data elements have been removed (HIPAA Privacy Rule).
- Names
- All geographic subdivisions smaller than a state, including street address, city, county, precinct, ZIP Code, and their equivalent geographical codes, except for the initial three digits of a ZIP Code if, according to the current publicly available data from the Bureau of the Census: a. The geographic unit formed by combining all ZIP Codes with the same three initial digits contains more than 20,000 people. b. The initial three digits of a ZIP Code for all such geographic units containing 20,000 or fewer people are changed to 000.
- All elements of dates (except year) for dates directly related to an individual, including birth date, admission date, discharge date, date of death; and all ages over 89 and all elements of dates (including year) indicative of such age, except that such ages and elements may be aggregated into a single category of age 90 or older.
- Telephone numbers
- Facsimile numbers
- Electronic mail addresses
- Social security numbers
- Medical record numbers
- Health plan beneficiary numbers
- Account numbers
- Certificate/license numbers
- Vehicle identifiers and serial numbers, including license plate numbers
- Device identifiers and serial numbers
- Web universal resource locators (URLs)
- Internet protocol (IP) address numbers
- Biometric identifiers, including fingerprints and voiceprints
- Full-face photographic images and any comparable images
- Any other unique identifying number, characteristic, or code, unless otherwise permitted by the Privacy Rule for re-identification
In addition, the submitting institution should have no actual knowledge that the remaining information could be used alone or in combination with other information to identify the individual who is the subject of the information.
2 Applicable Federal regulations may include HHS human subjects regulations (45 CFR Part 46), FDA human subjects regulations (21 CFR Parts 50 and 56), and the Health Insurance Portability and Accountability Act Privacy Rule (45 CFR Part 160 and Part 164, Subparts A and E).
3 For retrospective (older) studies where the participant consent form does not explicitly state broad data sharing, the IRB must determine whether the data can be submitted to NICHD DASH.